Formwork eQMS
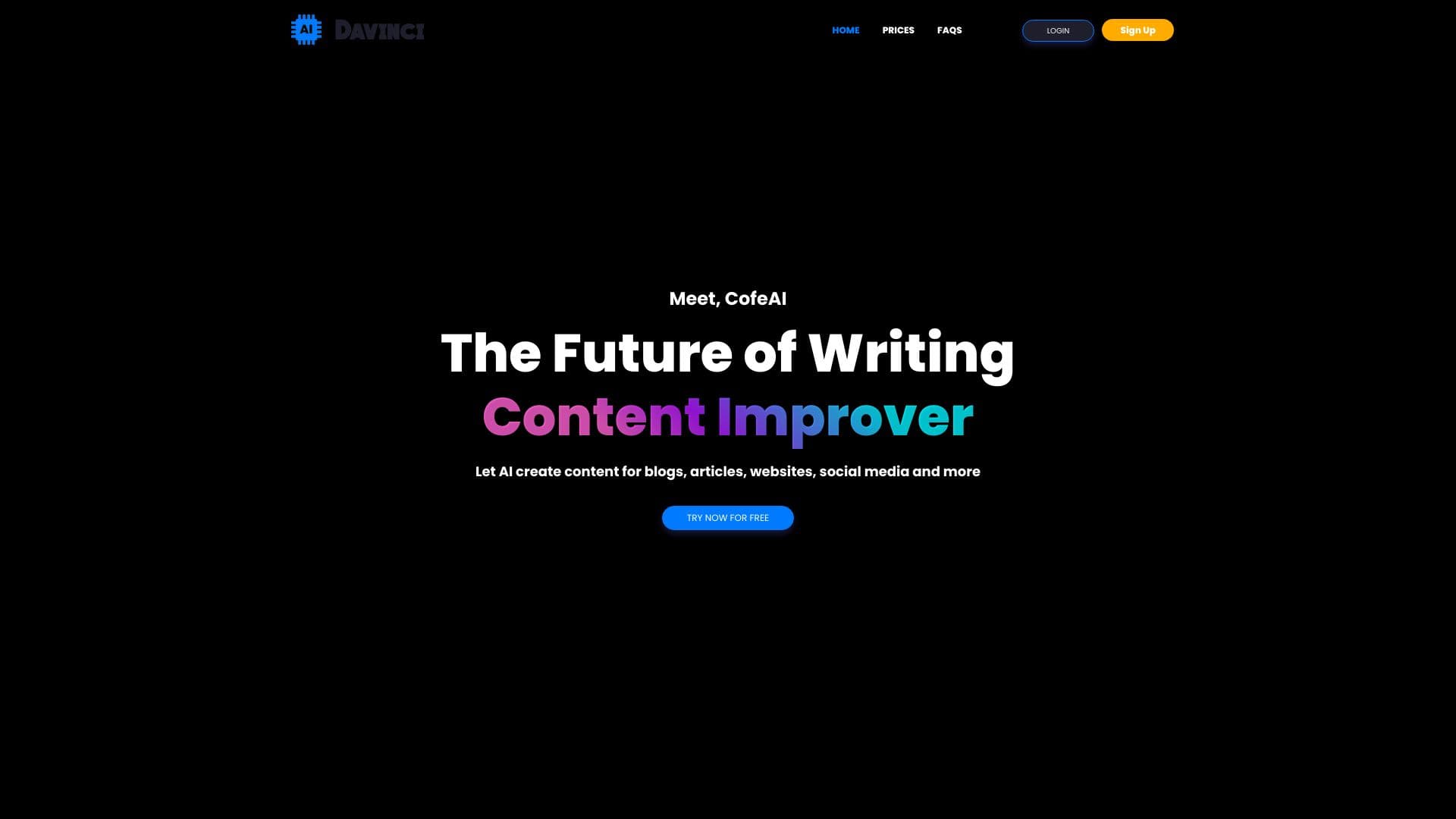
Formwork is a complete eQMS software and requirements management system for medical devices targeting EU MDR or US FDA approval. Its in-browser document and record editing features include a rich-text editor, document versioning and FDA CFR 21 Part 11 e-signing compliance. It also includes a user trainings feature to ensure that all employees are trained on relevant procedures. The requirements feature covers technical documentation as per IEC 62304, risk management as per ISO 14971 and usability evaluation as per IEC 62366.
Social Media
Formwork eQMS Introduction
What is Formwork eQMS?
OpenRegulatory is a medical device consultancy and SaaS provider headquartered in Berlin, Germany. It helps companies achieve compliance with the EU MDR and US FDA. In addition to consulting, OpenRegulatory offers Formwork, an eQMS software targeted towards agile, founder-led companies. Formwork is one of the most popular choices for eQMS software among medical device startups due to its unlimited user seats (no per-seat pricing), monthly billing (no commitments) and easy data export in case customers want to migrate to another provider.
How to use Formwork eQMS?
Visit website for more info
Why Choose Formwork eQMS?
Choose this if you’re in the medical device space and need a solid eQMS that helps with compliance, document control, and training. It’s especially handy for startups wanting flexible user seats and easy billing without long commitments.
Formwork eQMS Features
Features
Feature information not available.
Pricing
Starter
Formwork Starter is perfect for early-stage startups. Eligibility criteria are that companies don't have any revenue and funding yet, and consist of three full-time team members or less. It contains all features of higher-priced tiers: Full eQMS software for document and record management and requirements management for technical documentation.
- ✓ Unlimited user seats
- ✓ Unlimited documents
- ✓ EU MDR compliance
- ✓ US FDA compliance
Startup
All features of Formwork, at reduced price for startups. You're eligible for the Startup package if we can display your logo on our website.
- ✓ Unlimited user seats
- ✓ Unlimited documents
- ✓ EU MDR compliance